ARTICLE SUMMARY:
Hospital value analysis committees have supplanted clinicians’ traditional role as the gatekeepers for new product adoption in hospitals. Red Team Associates interviewed six VAC members of hospitals greater than 500 beds for their take on how medtechs need to craft the pitch for their innovative devices. By Harris Kaplan.
The days when selling a medical device meant cozying up to clinicians are gone.
 While clinicians continue to be a very important influence over which devices get used in a hospital, they're no longer the only ones with a seat at the decision-making table. At that table now is a diverse group of hospital administrators, care providers, and other stakeholders known as the Value Analysis Committee (VAC). It is this group that will decide what products get adopted.
While clinicians continue to be a very important influence over which devices get used in a hospital, they're no longer the only ones with a seat at the decision-making table. At that table now is a diverse group of hospital administrators, care providers, and other stakeholders known as the Value Analysis Committee (VAC). It is this group that will decide what products get adopted.
No matter how innovative a medical device company perceives its new product to be, if it’s being marketed to hospitals, chances are it will be evaluated by the VAC. The VAC will decide if the product gets used and the future commercial success of that new product hangs in the balance of their decision.
Hospitals formed VACs to seek a balance between cost reduction, more streamlined supply chain, and providing quality care in the purchase of medical devices and technology.
VACs: Balancing Care and Cost
As VACs sort through a barrage of new products that represent different levels of innovativeness (see below), they’ll try to balance the quality of care with costs. The VAC will focus on two broad questions that define the overall product’s value:
- What is the impact of the new product on improved patient outcomes?
- What is the impact of the new product on the cost of patient care?
Most VACs don’t worry about a product’s safety or efficacy, assuming the FDA has addressed those issues. Their concerns are if they approve the product how will it help patient outcomes and how will those outcomes impact the bottom line of the hospital? VACs will look closely at any and all published data, positive or negative. While positive endorsements and clinical data are very influential, negative product feedback, such as infection rates, readmission rates, or increased length of stay, product failure rates, adverse side effects, etc., may actually eclipse positive comments and data and can slow product adoption. VACs might decide to wait until there’s more experience and long-term data available before committing.
Clinician Advocacy: Necessary but Not Sufficient
Medtech companies continue to devote a tremendous amount of energy and money to convincing physicians of the value of their new product, and they do so for very good reason. Physician advocacy is a very important input to the VAC. Without it, a product has little chance of even being presented to the VAC.
But physicians are only one input. As the power of the VAC has grown and the requirements the VAC puts forth as needed to support product adoption, identifying physician product champions willing to back a new product has become a significant challenge for manufacturers. Reasons for this include:
- To a physician, it’s more work without more pay. Physicians have limited time available to assume this champion role and, for them to be effective in presenting to the VAC, it needs to be clear there is not a financial interest or compensation from the company manufacturing the product.
- A physician’s personal and professional influence is finite. They’ll use that power sparingly, and only champion products they really believe will make a significant difference in their ability to provide better care and outcomes.
- Over half of physicians are now hospital employees and part of their compensation is tied to their ability to keep costs down. So they may be far better aligned with the VAC than the manufacturer as far as supporting product adoption.
VACs: There’s Good News and Bad News for Manufacturers
First the bad news: The VAC’s power and influence over new product adoption has made it harder for companies developing new devices to get them adopted. The more disruptive the new technology and the higher its incremental cost, the greater the evidence the product manufacturer will have to provide as to the value of the new technology and the longer that evaluation is likely to take. Examples of questions that will need to be addressed by the manufacturer and the championing physician include:
- Does the new product replace a product or technology the hospital already uses?
- What benefits does this product offer that the hospital is not getting from what is currently being used?
- How much will the new device or technology improve the quality of care?
- How will the hospital measure that improvement?
- How long will it take before the hospital can see the benefits? The VAC members interviewed typically want to see meaningful financial or outcomes benefits within two years.
- How much does the device cost (up-front and ongoing) vs what the hospital is currently spending?
Now the good news: By the VAC making the new product acceptance criteria more difficult, they make it more challenging for later entrants to dislodge the incumbent. In essence, VAC acceptance creates the opportunity for a product to become the standard used throughout the hospital and, possibly, even the network if the hospital is part of a group system. It’s the equivalent of building a moat around the product and significantly increasing the products revenue and value.

Succeeding in the New Commercial Reality
There’s an argument to be made that the clinicians are really the only ones qualified to determine the value of a new product or technology. And many medtech companies still operate as if the clinician is both evaluator and decision maker.
That’s not the reality of how hospitals make purchasing decisions today. The role of the VAC is clear and it’s not going away. In fact, it’s growing.
Companies need to understand the various VAC members who will participate in the product adoption process and the perspective they will bring to the decision-making process.
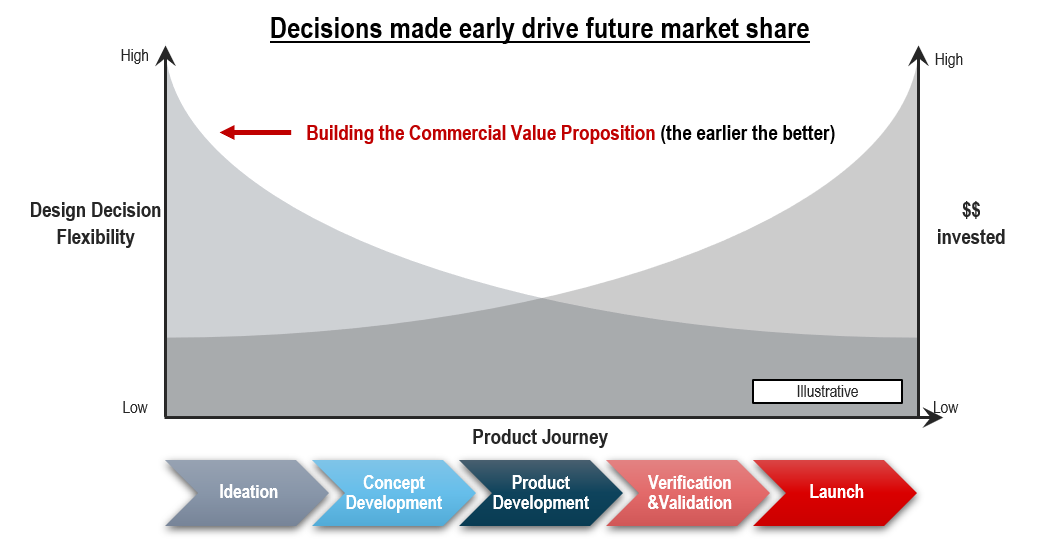
A Product’s Future Market Share is Won During the Design Phase
“Build it and they will come” is increasingly a footnote in the history of the medical device industry. In today’s more challenging commercial environment, the best time to anticipate the tough questions the VAC will ask and address them is during the product ideation and concept development phase. At that stage, the company still has the ability to modify the product design and hasn’t yet invested a lot of money in the product’s development.
Having direct input from VAC members as well as all others likely involved in the future purchasing process is both useful and smart business. The sooner engineers and scientists understand the customers’ unmet need, the better. The goal is to develop products that really address problems rather than just slightly improved versions of the products currently used.
Incremental product improvements may not justify a hospital’s wanting to switch products. Especially if the new product is more expensive and/or the current product is bundled in a broader portfolio contract.
VAC: The Elephant in the Room
No doubt, the growth of the influence of the VAC has made commercial success and quick new product adoption more difficult to achieve.
Are VACs sucking the life out of product innovation? Not in the least, but they have raised the innovation bar for new medical devices.
Companies who build the VAC’s concerns into their new product development will have an easier time achieving commercial success. And, as already stated, the products approved and utilized will have a natural moat around their revenues as any competitor attempting to dislodge them will have to attain an even higher innovation baror be willing to cut their price significantly to justify making the product switch.
Harris Kaplan (harris.kaplan@redteamassociates.com) is Managing Partner of Red Team Associates and the founder and CEO of Healogix, both life sciences consultancies based in Horsham, PA.
![]() Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
