ARTICLE SUMMARY:
Healthcare systems around the world are, for the most part, delivering care that fails to help significant portions of the patient population. Personalized medicine is poised to detect the onset of disease at its earliest stages, preempt the progression of disease, and increase the quality, accessibility and affordability of care.
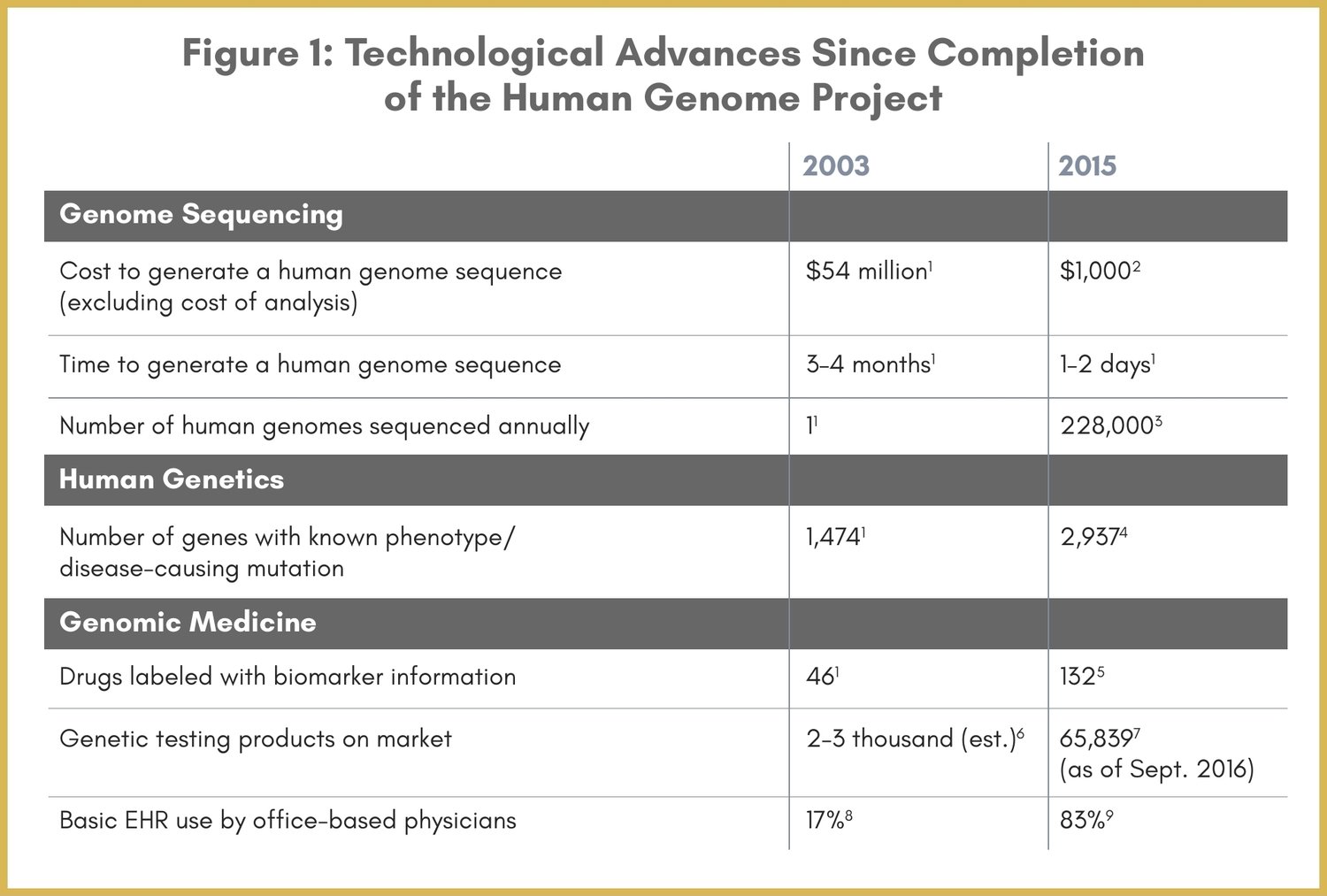
“One size fits all” is a concept that doesn’t work too well with clothing, and it isn’t an effective solution for today’s overburdened global healthcare systems either. Enter personalized medicine. Remarkable innovations in genetic sequencing technology, brought about since the completion of the Human Genome Project in 2003 (see Figure 1; also see our May 21, 2019 Community Blog post, “In MedTech History: The Human Genome” ), are allowing disease treatment to begin to be tailored so that differences in people's biological makeup is taken into account. The goal is to target the right treatments to the right patients at the right time – helping to shift the emphasis in medicine from reaction to prevention, direct targeted therapy, reduce adverse drug reactions, reduce invasive testing procedures such as biopsy, and control the overall cost of healthcare. In just one example of many, patients with a variety of cancers routinely undergo molecular testing as part of patient care, enabling physicians to select treatments that improve chances of survival and reduce exposure to adverse effects.

The Community Blog spoke with Christopher Wells, Vice President of Public Affairs for the Personalized Medicine Coalition (PMC), to see where the medtech industry and the FDA now stands in terms of personalized medicine, and where the field is headed. PMC represents all sectors of the healthcare system to encourage investment in and adoption of advanced personalized medicine products. It works closely with policymakers at the Centers for Medicare and Medicaid Services (CMS) and the FDA, among other US as well as international agencies, to advocate for policy decisions that support the adoption of personalized medicine, and educate clinicians, patients, payers, employers and others on the benefit of tailored therapeutics (a critical challenge that PMC is looking to address: developing the clinical and economic evidence needed for reimbursement and patient access).
“Personalized medicine represents an extraordinary opportunity for the health system largely because of the proliferation of medical devices that make it possible to target treatment to only the patients who will benefit from it,” Wells tells the Community Blog. “The diagnostics industry, in particular, is working hard to ensure that the value proposition for personalized medicine is recognized … so that these diagnostic tools can guide therapies,” he continues.
The FDA does seem very committed to advancing personalized medicine forward in the US, Wells says. In late 2015, the agency launched precisionFDA, an online, cloud-based, crowd-sourced portal that will allow scientists from industry, academia, government and other partners to come together to foster innovation and develop the science behind next-generation sequencing (NGS). As of this month, the precisionFDA community includes nearly 5,000 users across 1,200 organizations, with more than 38 terabytes of genomic data stored.
This past March, personal genetics company 23andMe Inc. received the first-ever FDA authorization for a direct-to-consumer genetic test for cancer risk. The authorization allows 23andMe to provide customers, without a prescription, information on three genetic variants found on the BRCA1 and BRCA2 genes known to be associated with higher risk for breast, ovarian and prostate cancer. Then in April, the FDA issued two final guidances that recommend approaches to streamline the submission and review of data supporting the clinical and analytical validity of NGS tests.
Looking ahead, scientific developments in areas such as genomic sequencing, immunotherapy, gene therapy, and CRISPR-Cas9 gene editing, as well as advances in health information technology are laying the groundwork for a new era in medical discovery and more efficient, personalized treatment, according to PMC.
“The challenge right now is to catch up to the possible,” Wells tells the Community Blog. Trends on the horizon in personalized medicine include artificial intelligence (AI) and the role that that might play in uncovering the relevant biomarkers and health data that we should think about in order to make care even more personalized, he says. That dovetails with the opportunity in real-world evidence (RWE), to be able to look at data from actual clinical practice settings and personalize treatment strategies according to that. There is a lot of opportunity and enthusiasm around both of these key trends, says Wells.
#CommunityBlog #MedTechStrategist #HumanGenomeProject #PersonalizedMedicineCoalition #CMS #FDA #23andMe #precisionFDA #personalizedmedicine #precisionmedicine #nextgenerationsequencing#artificialintelligence #realworldevidence #medicaldevice #medtech #tracyschaaf
![]() Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
