ARTICLE SUMMARY:
The gap between the availability of ventilators and projected demand is high, with estimates varying depending on whether the COVID-19 pandemic is mild, moderate, or severe. GlobalData has some very rough estimates of demand.
COVID-19 has highlighted just how completely under-prepared global healthcare systems are for large public health crises and how stressed are the critical care and protective medical equipment supply chains. The category of devices most in the spotlight certainly has been respiratory ventilators, which for a variety of reasons are in very short supply. As the US and other governments look for ways to ramp up supplies, it is not at all clear how easily this can be done, or whether it will translate into commercial opportunities for traditional manufacturers, many of which were already producing ventilators and protective medical gear at full capacity.
To better evaluate global capabilities and options for expanding ventilator supply, it is worthwhile to step back and outline the status of the sector prior to COVID-19. To do this, MedTech Strategist discussed the situation with GlobalData medical device analysts Andrew S. Thompson, PhD, director of therapy research and analysis, medical devices, and Tina Deng, senior analyst, medical devices. They provided pre-COVID-19 data on ventilator demand, as well as crude estimates of how COVID-19 is likely to shift those numbers.
Deng notes that estimates are determined based on mild, moderate, and severe projections of local modeling of the disease, amid a rapidly changing situation. The projections were published on March 17, when the crisis was well underway in parts of Europe but had not hit the US in full force. As such, they are very rough, but provide at least a toehold which can be used to assess the situation.
Prior to the COVID-19 outbreak, GlobalData valued the global respiratory ventilator market at $1.4 billion in 2019, or 74,606 units, including both critical care and transport equipment. The compounded annual growth rate (CAGR) was steady at 4%, driven by an aging population, an increasing number of intensive care unit (ICU) admissions, and expanding demand for respiratory devices in emerging countries.
The other key question in this fight is manufacturing capacity and supply chain resilience. GlobalData analysts say while their data has provided insights for designers of emergency equipment, they cannot discuss manufacturing capacity,as many companies are now involved in negotiations with their respective governments about the construction of very basic but serviceable transport ventilators. These use commonly available, indigenously produced components, and differ significantly from protocols of traditional, regulated manufacturers, which use more custom components. But uncertainty over the ability of these new players to jump into the field rapidly makes it hard to estimate production capacity.
The traditional ventilator manufacturers estimate that they could double capacity, although it’s not clear how rapidly. To do this, they need to hire staff and expand assembly lines (though this is restricted by the availability of clean rooms). They may also face supply limits of complex parts, Thompson said in an email.
The “emergency” ventilators made by car makers and other, non-traditional manufacturers won’t be approved by the regular channels, as there is no time to do that, he adds. Instead, they will have to meet a minimally acceptable standard and likely after COVID-19 subsides, they will be scrapped. Volume may not be an issue if car manufacturers are mandated to contribute to the production, as car plants are currently sitting idle.
More insights will be forthcoming from the ventilator manufacturers. Draegerwerk, a German company that is one of the world’s leading manufacturers of ventilators, for example, had recently reorganized its medical products business and was in the early stages of launching a new line of ventilators in Europe with secure, state-of-the-art network connectivity when the coronavirus surfaced as a threat in China. On a March 5 earnings call, its Chair Stefan Draeger pointed to the limited ability of his firm to increase production capacity on very short notice, as it is already operating at full capacity. The biggest hurdles to capacity expansion are supply chain issues (it has two manufacturing plants in China), and shortages of testing equipment. If the company keeps pricing stable, it still faces increased freight expenses, he continued.
What follows is based on an email exchange with Tina Deng.
MedTech Strategist: What is the global manufacturing capacity for respiratory ventilators?
Tina Deng: The ventilator manufacturers have full order books now. Based on what I read, most manufacturers aim to more than double their capacity to meet the needs not only from regular customers like hospitals, but also directly from governments.
What are the safety standards for minimally acceptable ventilators that will be produced by both seasoned medical device companies and those new to the field as a response to the COVID-19 pandemic? You have previously written on safety issues and recalls of ventilator manufacturing.
The majority of ventilators that are made specifically for the COVID-19 outbreak are likely to meet minimally clinically acceptable standards, but will not be advanced ventilators with a complicated variety of modes and options. The simple design not only makes it easy to manufacture in maximum capacity, but also largely shortens the training time.
According to the UK government, these rapidly manufactured ventilator systems will not be CE marked and not recommended for routine care. They require less than 30 minutes of training for a doctor with some experience.
Additionally, guidance issued by the UK Medicines and Healthcare Regulatory Agency (MHRA) (Rapidly Manufactured Ventilator System RMVS001), which specified options for clinically acceptable ventilators to be used in UK hospitals during the COVID-19 outbreak, specifically highlighted the need for military electronic engineering experience with respect to providing a reliable power supply.
Do you have insights into how hospitals generally manage ventilator inventory under normal conditions—are there government regulations governing inventory requirements?
I'm not aware of any specific government regulations governing numbers. But ventilators are essential equipment in the ICU. Today’s ventilators are common in hospitals, long-term care centers, emergency centers, and even patients’ homes to save hundreds of thousands of lives every day by providing respiratory ventilation.
Have your projections on market size and growth shifted in light of the current pandemic?
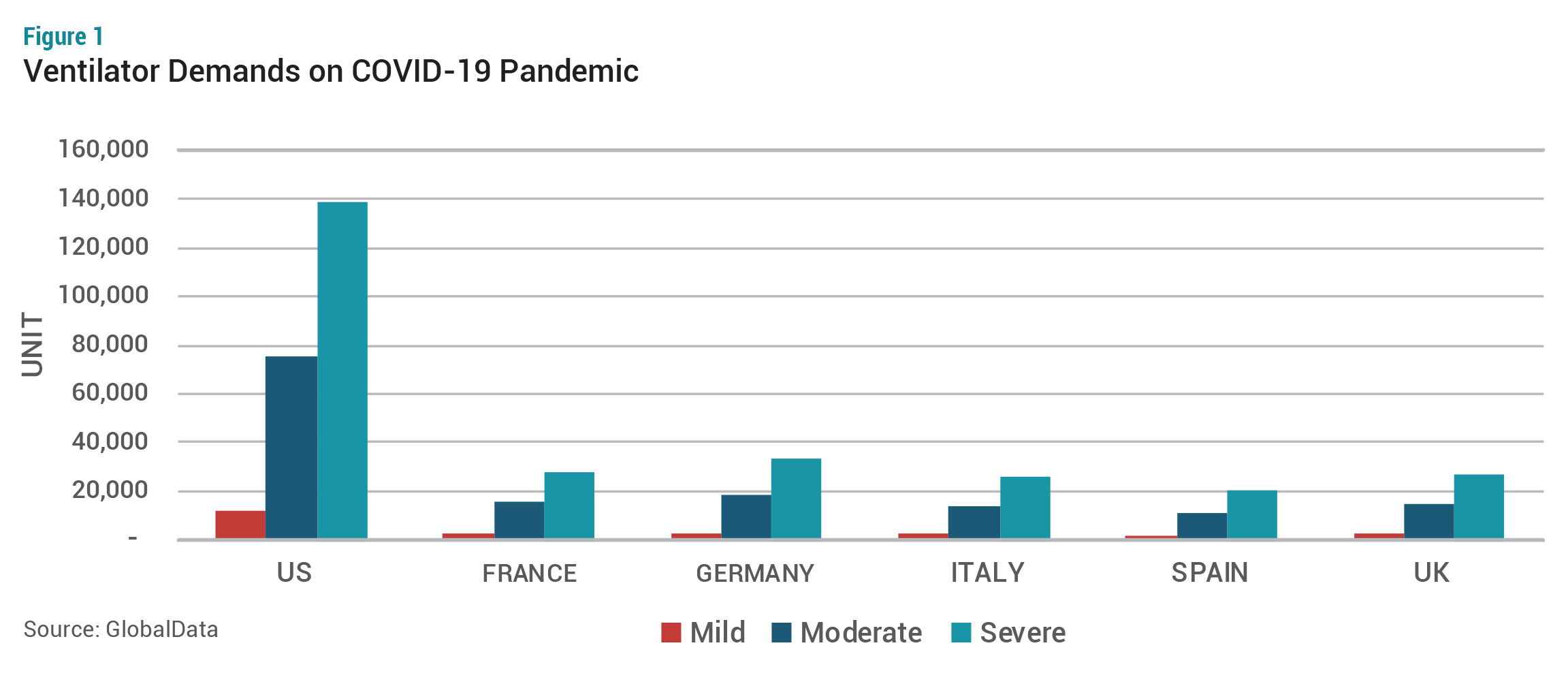
The situation is changing rapidly. On March 17, we estimated approximately 880,000 more ventilators were in demand globally due to the COVID-19 outbreak. The estimation has been derived based on the mild, moderate, and severe pandemic scenarios. In a moderate COVID-19 pandemic, the US has a gap of 75,000 ventilators, while four EU countries (France, Germany, Italy, Spain) and the UK lack 74,000 ventilators (see Figure 1). GlobalData describes mild pandemic as similar to the 1957 and 1968 influenza pandemics. Severe pandemic is defined as the situation in the 1918 influenza pandemic.
Will anyone profit from ventilator manufacturing scale-up?
The current ventilator manufacturers will enjoy profit from the massive orders. The new market disrupters such as non-medical engineering companies and car makers will have the opportunity to step into the medical device field.
Who are the top respiratory ventilator manufacturers ranked by sales for US and/or global sales?
Prior to the COVID-19 outbreak,Draegerwerkand Maquet (Getinge) are leaders in the global market, followed by Medtronic plc and Hamilton Medical. The largest and most dynamic companies in the ventilation equipment market offer comprehensive portfolios of critical care ventilators, transport ventilators, and associated accessories and consumables. They dominate the market by offering a variety of products with extensive feature sets in a different area. Other companies include Air Liquide, CareFusion, Fisher & Paykel Healthcare, General Electric, Philips, ResMed, and Smiths Medical.
![]() Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
*End of Article*