ARTICLE SUMMARY:
Eclipse Regenesis is developing a pediatric device for the restorative treatment of short bowel syndrome. In March 2020, the team was selected as the winner of the UCSF-Stanford Pediatric Device Consortium Accelerator competition. Health Advances interviewed two of the company founders to discuss the development of their mechanical solution and advice for pediatric entrepreneurs. By Andrew Millar, Juliana Perl, and Susan Posner, Health Advances.
About 18 months ago, Medtech Strategist featured an article highlighting the unique challenges of pediatric device development.(See “The Challenge of Pediatric Device Development,” MedTech Strategist, January 30, 2019.) Key challenges include: both smaller sized and fewer patients relative to adults, the need for devices that adapt to growing bodies, and significant heterogeneity between pediatrics of different ages, all of which present barriers to developing and commercializing new technologies. Given these barriers, many pediatric patients face unmet needs related to the devices available to them today. For over seven years, FDA has recognized this need, and has funded a number of Pediatric Device consortia to encourage innovation for pediatric patients. The consortia do this by providing both funding and mentorship for early-stage device innovators. As part of an ongoing effort to highlight this need and pediatric innovators more broadly, we sat down with the innovators behind Eclipse Regenesis Inc., a start-up with a novel solution for short bowel syndrome.
For patients with short bowel syndrome (SBS), the small intestine is not long enough to allow for the proper absorption of nutrients. This condition can result from either surgical removal of part(s) of the intestine, or in the case of many pediatric patients, a congenital condition. SBS is very rare, and while its exact incidence is unknown, one study estimated an incidence of 24.5 per 100,000 live births. Today, patients affected by this condition rely on non-curative treatment such as total parenteral nutrition (TPN), pharmaceuticals, or transplantations that can also be difficult to afford in some cases. Unfortunately, pediatric patients still face up to 40% mortality rates by age three.
Eclipse Regenesis founders Andre Bessette, James Dunn, MD, PhD, and Tom Krummel, MD, are working to develop the first restorative solution for pediatric SBS patients. Their device features a mechanical spring that works to internally grow new, healthy tissue to lengthen existing segments. As of June 2020, the device saw successful animal models with generation of tissue seemingly identical to that of the existing healthy intestine.
In our interview with Mr. Bessette and Dr. Dunn, the team describes their future endeavors to expand the tissue growth mechanism to various hollow organs throughout the body, the path to commercialization, and what it takes to bring a pediatric device to market.
Health Advances: Where did Eclipse Regenesis, and the idea behind it, get its start?
 James Dunn: It started after I moved to Stanford. I was at UCLA for 15 years and a lot of the work on this device originated there. When I moved to Stanford, a variety of seed grants helped spark the genesis of this company. Tom Krummel has been in this space for a long time and understands this issue well, as he’s a pediatric surgeon too. Together we applied and received the Coulter Foundation grant in order to start the company. On the particular day of the great eclipse across the United States, we were able to get everything started, so we decided to call it Eclipse.
James Dunn: It started after I moved to Stanford. I was at UCLA for 15 years and a lot of the work on this device originated there. When I moved to Stanford, a variety of seed grants helped spark the genesis of this company. Tom Krummel has been in this space for a long time and understands this issue well, as he’s a pediatric surgeon too. Together we applied and received the Coulter Foundation grant in order to start the company. On the particular day of the great eclipse across the United States, we were able to get everything started, so we decided to call it Eclipse.
 Andre Bessette: I got involved through Tom Krummel. We have been working together almost 15 years through different companies and projects. We were first introduced and worked together on a spin off technology out of Stanford called PEAK Surgical. PEAK was acquired by Medtronic. Since then, Tom and I worked on several different projects. When I saw this opportunity, James was moving to Stanford, I was an entrepreneur-in-residence at Lagunita Biosciences, which is an incubator and fund, and Tom is on the board there. So it all came together at the right time.
Andre Bessette: I got involved through Tom Krummel. We have been working together almost 15 years through different companies and projects. We were first introduced and worked together on a spin off technology out of Stanford called PEAK Surgical. PEAK was acquired by Medtronic. Since then, Tom and I worked on several different projects. When I saw this opportunity, James was moving to Stanford, I was an entrepreneur-in-residence at Lagunita Biosciences, which is an incubator and fund, and Tom is on the board there. So it all came together at the right time.
How do you see Eclipse filling the need of a restorative solution, and is there a patient group best suited for the treatment?
Dunn: I think the patients who will benefit most from this approach are kids with very short, ultra-short amount of intestine. Many of the short gut patients have enough intestine that, given enough patience and barring bad complications, they can come off the intravenous support after some years. But for ultra-short gut patients, there is less hope. These children are dependent on intravenous nutrition and suffer from complications. We have seen many kids who received intestinal transplantation, but they suffer from a new set of problems introduced by the treatment. Thinking about alternative solutions to what today is just supportive care, is something that I have pondered throughout training, and then I came up with this idea.
How did you get the idea to take this approach?
Dunn: Specifically, I think it can be traced to a Grand Rounds where there was a talk about osteogenesis, where forces applied to bone can lengthen it. That sparked the idea that if you can do this to bone, why can’t you do it to the intestine? Of course, that is not the only example. In surgery we use tissue expanders for reconstruction all the time. But those did not light the idea until I heard this particular talk, and I remember distinctly sitting in the audience and thinking, “This has got to be applicable to the intestine as well.”
Other than supportive care, is there anything we can do for these patients today from the surgical perspective?
Dunn: Yes, from the surgical perspective, besides the transplant patients that I mentioned, there are surgical methods to make the intestine longer. The most recent iteration of that is called a STEP procedure, which stands for Serial Transverse Enteroplasty. Which essentially means taking a piece of intestine and cutting it in such a way that it is longer. The surgeon makes certain cuts in a dilated piece of intestine and then reconfigures that into narrower pieces of intestine. It seems like a great idea, but it is not very widely accepted. The biggest problem I see is that you do not really increase the amount of intestine, it is reconfigured. So total absorption is the same and there are some complications associated with it.
Besides surgical approaches, there are also medical approaches that seem to enhance absorption. Again, not a cure. It does not increase the length of the intestine, but it does enhance the function of the intestine in some of the patients. So it is used in addition to supportive intravenous therapy.
What do you see as the biggest challenges you have faced so far in development and commercialization?
Bessette: One of the biggest challenges is that this has never been done before. This is unique. And I think it’s exciting and there is great possibility with a unique solution, but with that comes a lot of the unknowns when you are speaking with investors and to physicians. Getting people to really understand the clinical and economic need, and what the solution can bring, poses challenges. I think James’ design, and the device itself, is very elegantly simple in its approach. The mechanical solution to stimulate tissue growth is proven in orthopedics with bone lengthening and in plastic surgery with skin expansion. So that part of it, the mechanism makes sense, the science is solid. But when you show something and talk about something that people have not seen before, many times the first reaction is that you are crazy or good luck with that. That impacts fundraising. But I think with a great team, great story, and great science, you will find the right investors that believe and understand the need. They will see that these pediatric patients and society will benefit tremendously by this.
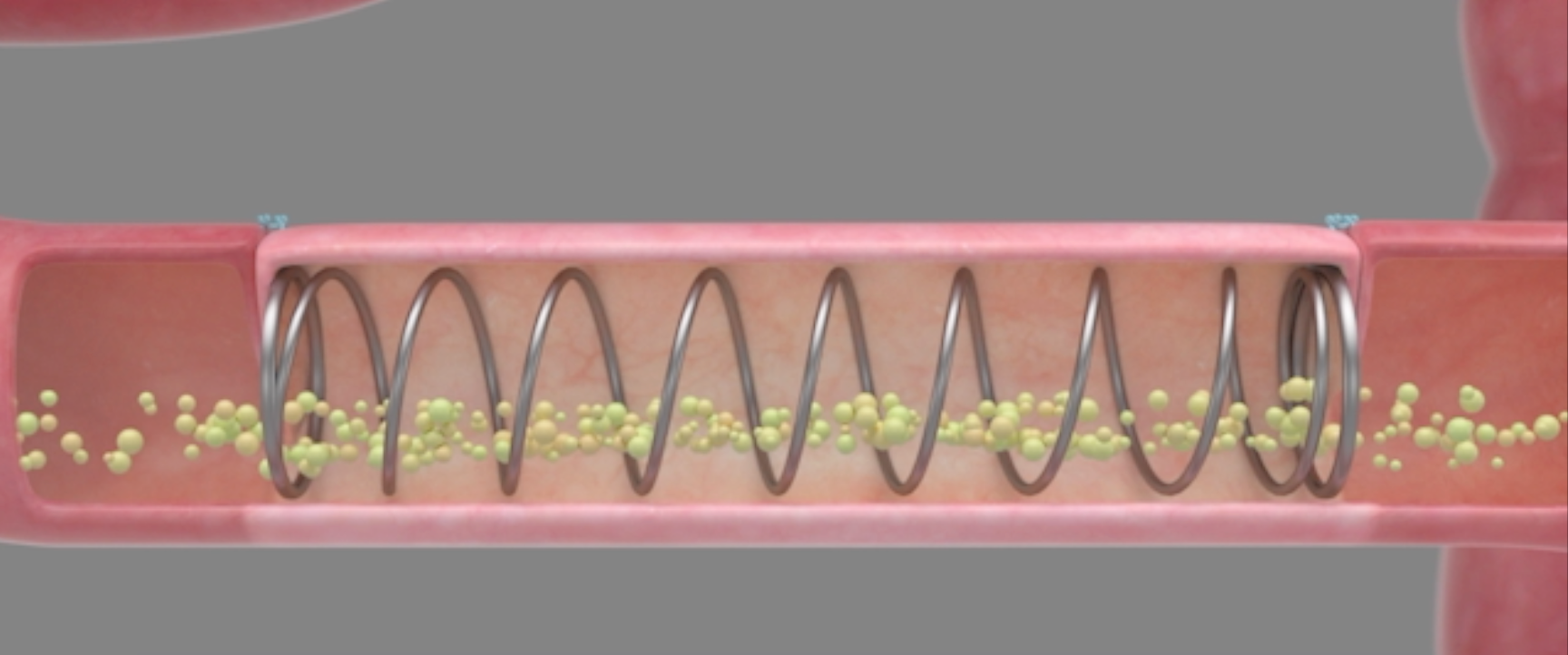 On the Eclipse XL 1 device itself, the design and the development has gone very smoothly because of its mechanical nature; it is a nitinol device, with memory shape, which is a proven technology (see product photo). We are working on delivering it to the right place, getting it to remain in the intestine and perform its job, which is going well but it is not without its challenges. That of course rolls into the regulatory side too. There are challenges there too, and our goal right now, is to do the first in-human application of this. It had great success in the large animal model, but translating that, making that leap, that first step to humans, it is a big step.
On the Eclipse XL 1 device itself, the design and the development has gone very smoothly because of its mechanical nature; it is a nitinol device, with memory shape, which is a proven technology (see product photo). We are working on delivering it to the right place, getting it to remain in the intestine and perform its job, which is going well but it is not without its challenges. That of course rolls into the regulatory side too. There are challenges there too, and our goal right now, is to do the first in-human application of this. It had great success in the large animal model, but translating that, making that leap, that first step to humans, it is a big step.
Can you compare and contrast what the challenges are relative to companies of your size that might be developing a device for the adult population?
Bessette: Right off the top, many investors will dismiss you because the market is too small. They will ask, “How many patients? How many surgeries per year? No, not interested.” That happens when you compare pediatrics to adults. But on the flip side, you also find compassionate investors that see the benefit of addressing the pediatric patient population. They want to do good, and they want to put their money to doing something good. It’s about finding the right investors. Then obviously it comes down to the investment environment. We were fortunate. We raised money back in 2019, times have changed now but we will see what the future brings.
In the same line of gaining momentum, how will you use the UCSF-Stanford Pediatric Device Consortium competition funding?
Bessette: The $50,000 PDC funding was great to win. That will be used for completing the development of our human use units that we will use it in our first in-human clinical trial.
When do you anticipate starting the trial?
Bessette: COVID has changed timelines but we are hopeful the first in-human trial will begin in 12 to 18 months.
While the device is intended to treat kids with short gut, it is using the principle of mechanical force induced growth. So it can be applied to a variety of organs.
What do you see as the broader future for this device?
Dunn: While the device is intended to treat kids with short gut, it is using the principle of mechanical force induced growth. So it can be applied to a variety of organs. For example, the esophagus is another area where babies are born with the esophagus not connected, esophageal atresia. This device could be modified and applied to the esophagus so there will be esophagus available for the surgeon to connect the two ends. I think that will lead to a significant paradigm shifts on how we manage that condition and others like it. You could potentially apply this to every hollow organ in the body such as the bladder, stomach, colon, vagina.
Bessette: It is interesting to note that the core patent does cover the concept of a hollow viscous organ in the body and a spring being able to stimulate growth. It has broad applications, not only in pediatrics but also in adults. That is part of our vision for the device, technology, and growth of the company. Both pediatric and adult. Unlike many companies that move from adult to pediatrics, we are actually starting in pediatrics and eventually we will move into adults.
What advice would you offer to other early stage innovators looking to innovate in pediatric devices?
Bessette: My advice would be do not listen to the nay-sayers. Continue to knock on doors and work through your network to find people that will understand your story and be motivated to invest and support the project. It may come from different areas. It may not be traditional medtech investors. They are out there, you just have to find them. You need to stay strong and keep moving.
Dunn: I agree with those sentiments. I tell people about the 5 P’s that I have experienced and I think are important in any of these endeavors. You have to have the right preparation. Then you need to have the passion for the work. It has to be a burning thing in your heart to want to do this. It takes a lot of hard work – which creates the next P, perspiration. Many hours uncompensated, probably unappreciated, are needed to make it happen. And then partnership. Finding the right people in the right environment is absolutely key. The last thing is what Andre said, perseverance. You have to keep going.
Andrew Millar is an Engagement Manager at Health Advances, leading project teams for medical device companies and investors in Health Advances’ Boston area office. Juliana Perl is pursuing a Master’s in Engineering Management at Stanford after working as a Senior Analyst in the Health Advances San Francisco office. Susan Posner is a Partner at Health Advances and a leader of the MedTech practice. Susan has over 20 years of health care experience. She can be reached at sposner@healthadvances.com.
Health Advances MedTech Practice supports medical device companies and investors in evaluating and executing upon commercial opportunities, both organically and through M&A.
![]() Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
