ARTICLE SUMMARY:
New guidelines on clinical investigations and unique device identification as Europe readies for the May 26 Medical Device Regulation go-live.
Clinical investigation Q&A. A bigger emphasis on clinical investigations is a core feature of the MDR; EU policymakers are hoping to further clarify those requirements in a new Q&A guidance on clinical investigations under the MDR. The 28-question document addresses the different regulatory pathways for device trials that are applied depending on the circumstances, safety reporting from trials, and how to handle “substantial modifications” to trials, among other topics. It lays out alternative pathways for clinical investigations in a flow chart:
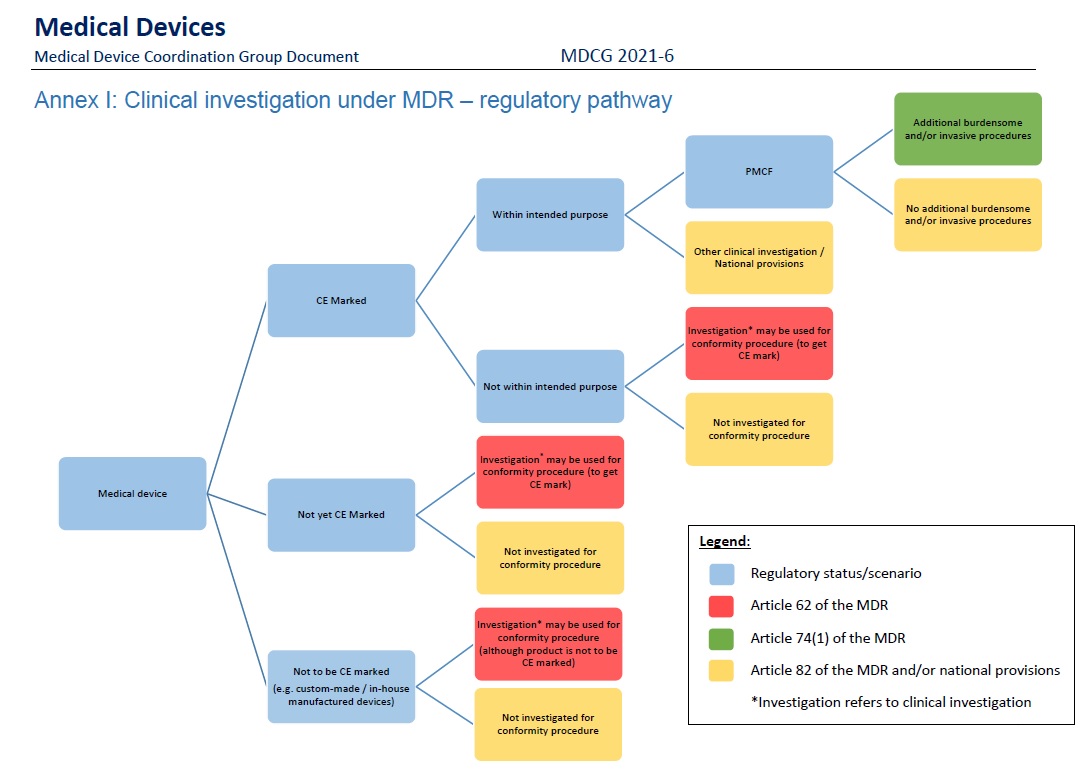
UDI updates. MDCG also recently updated its guidance on “Basic-UDI-DI and changes to UDI-DI” to explain companies’ responsibility to disclose the “maximum number of reuses” of a reusable device in the EUDAMED unique device identification database. The MDR requirement includes an “if applicable” proviso, which MDCG says means that the mandate is only for devices where a company has clinical evidence and a risk management process that demonstrates a specific number of reuses that should not be surpassed. If the maximum number of reuses changes, it will require a new UDI-DI, the guidance update states. Several more documents focused on implementation of the EU’s UDI system are expected out soon, including one on integrating UDI into companies’ quality management systems, another that may align some technical UDI practices with International Medical Device Regulators Forum best practices, and specific UDI rules for contact lenses.
More to come. Those additional UDI documents and other MDR implementation resources are likely to roll out in the days following the May 26 date of application for the MDR; the MDCG has its next full group meeting scheduled for May 27-28, when it is likely to endorse an array of items to support the new regulation. Other steps and items that could come from that gathering: at least one more MDR notified body designation (for a total of 21), additional steps to fully adopt the new European Medical Device Nomenclature, based on Italy’s Classificazione Nazionale Dispositivi medici, including a document listing harmonized terms that should be included on patient implant cards, and more guidances to support compliance to MDR postmarket surveillance requirements.
Excerpted from “Pathways’ Picks April 28: New Tech Payments, MDR Watch, Saudi AI Guide, and More,” Market Pathways, April 28, 2021.
Trial MyStrategist.com and unlock 7-days of exclusive subscriber-only access to the medical device industry's most trusted strategic publications: MedTech Strategist & Market Pathways. For more information on our demographics and current readership click here.
